Chemicals Expiry Date . when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. expiration date is limited to the date listed by the manufacturer. a chemical might undergo chemical changes due to different storage conditions: for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. It could react with other chemicals present in the air or in the. the fda has published a q&a document regarding the establishment of expiry dating for chemicals, reagents,.
from www.dreamstime.com
expiration date is limited to the date listed by the manufacturer. for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. It could react with other chemicals present in the air or in the. when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. the fda has published a q&a document regarding the establishment of expiry dating for chemicals, reagents,. a chemical might undergo chemical changes due to different storage conditions:
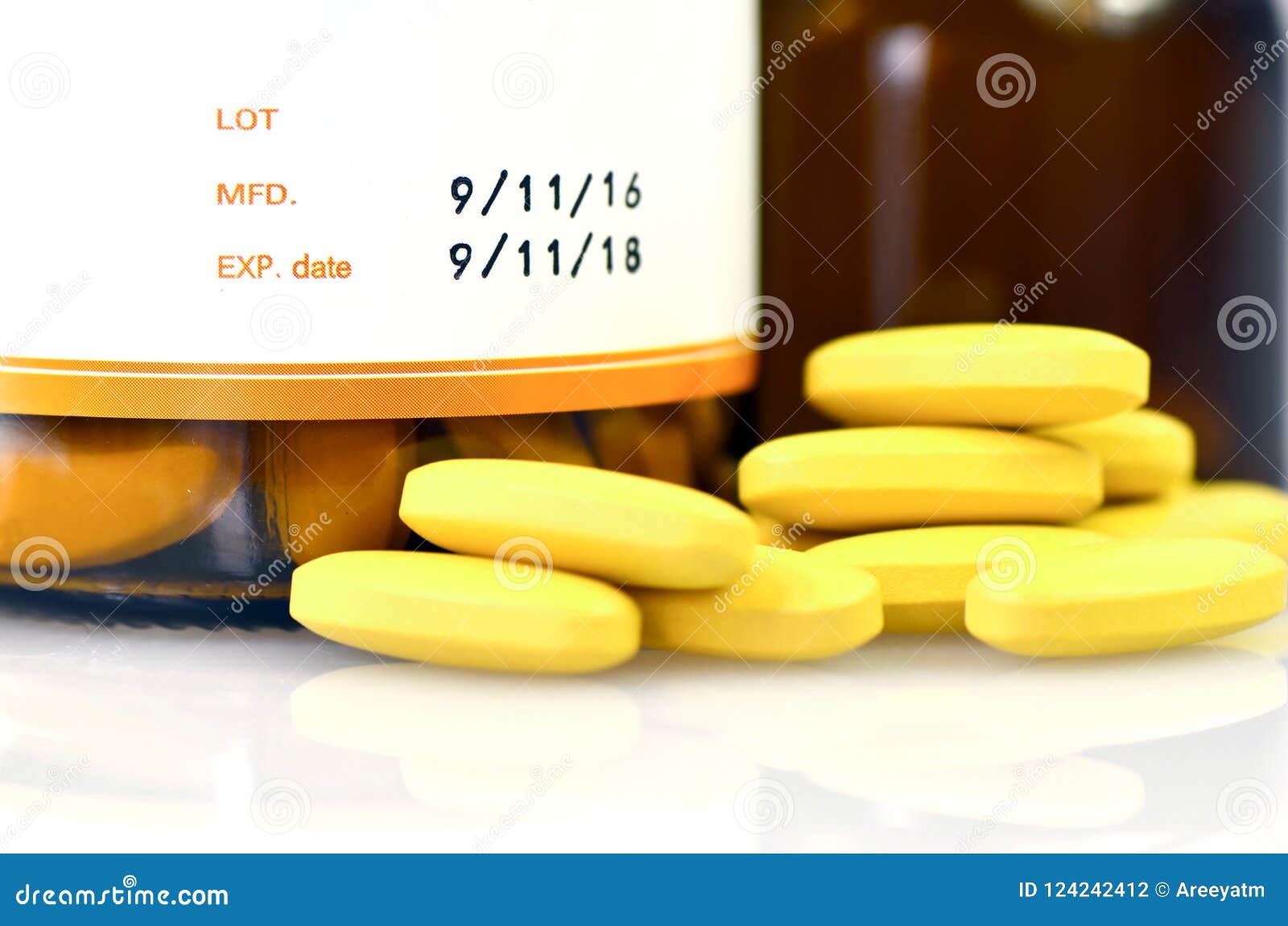
Manufacturing Date and Expiry Date on Some Pharmaceutical Packaging. Stock Photo Image of
Chemicals Expiry Date when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. It could react with other chemicals present in the air or in the. expiration date is limited to the date listed by the manufacturer. a chemical might undergo chemical changes due to different storage conditions: for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. the fda has published a q&a document regarding the establishment of expiry dating for chemicals, reagents,.
From www.vecteezy.com
expiry date label 13355649 Stock Photo at Vecteezy Chemicals Expiry Date a chemical might undergo chemical changes due to different storage conditions: It could react with other chemicals present in the air or in the. when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. for those materials where shelf life information is a requirement,. Chemicals Expiry Date.
From www.enfamil.com
Expiration Dates, Lot Numbers & Batch Codes Enfamil Chemicals Expiry Date when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. expiration date is limited to the date listed by the manufacturer. for those materials. Chemicals Expiry Date.
From www.youtube.com
How to Track Chemical Inventory Expiration Dates in RealTime YouTube Chemicals Expiry Date the fda has published a q&a document regarding the establishment of expiry dating for chemicals, reagents,. expiration date is limited to the date listed by the manufacturer. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. when there is low risk of product degradation due to. Chemicals Expiry Date.
From laptrinhx.com
Medicine Expiration Dates Do They Matter LaptrinhX / News Chemicals Expiry Date It could react with other chemicals present in the air or in the. expiration date is limited to the date listed by the manufacturer. the fda has published a q&a document regarding the establishment of expiry dating for chemicals, reagents,. expiration date refers to the amount of time an opened reagent will last before needing to be. Chemicals Expiry Date.
From bitesizebio.com
An Easy Guide to Chemical Expiry Dates Top 10 Lab Chemicals Chemicals Expiry Date It could react with other chemicals present in the air or in the. a chemical might undergo chemical changes due to different storage conditions: expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. for those materials where shelf life information is a requirement, expiration and retest periods. Chemicals Expiry Date.
From nickmorel3.blogspot.com
gluminal How to Read Expiration Dates Chemicals Expiry Date expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. It could react with other chemicals present in the air or in the. when there is low risk of. Chemicals Expiry Date.
From www.thelawnforum.com
Expiration Dates on Lawn Chemicals...A Mystery? Lawn Care Forum Chemicals Expiry Date for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. It could react with other chemicals present in the air or in the. the fda has published a q&a. Chemicals Expiry Date.
From www.labmanager.com
How to Handle Lab Reagents After Their Expiration Date Lab Manager Chemicals Expiry Date the fda has published a q&a document regarding the establishment of expiry dating for chemicals, reagents,. when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. expiration date refers to the amount of time an opened reagent will last before needing to be disposed. Chemicals Expiry Date.
From kmph.com
The truth about expiration dates KMPH Chemicals Expiry Date expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. a chemical might undergo chemical changes due to different storage conditions: for those materials. Chemicals Expiry Date.
From healthservice.hse.ie
Chemicals healthservice.ie Chemicals Expiry Date expiration date is limited to the date listed by the manufacturer. It could react with other chemicals present in the air or in the. a chemical might undergo chemical changes due to different storage conditions: expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. when there. Chemicals Expiry Date.
From www.studocu.com
Science lab Lab equpment LABORATORY INVENTORY Chemicals Name Quantity Expiry date Aluminum Chemicals Expiry Date when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple methods to assess the. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. expiration date is limited to the date listed by the manufacturer. It could react with. Chemicals Expiry Date.
From www.dreamstime.com
Macro Expiration Date on Canned Food Stock Image Image of metallic, date 51657379 Chemicals Expiry Date the fda has published a q&a document regarding the establishment of expiry dating for chemicals, reagents,. It could react with other chemicals present in the air or in the. expiration date is limited to the date listed by the manufacturer. a chemical might undergo chemical changes due to different storage conditions: when there is low risk. Chemicals Expiry Date.
From www.pharmaguideline.co.uk
Chemicals and Reagents Management in Quality Control Laboratory Chemicals Expiry Date a chemical might undergo chemical changes due to different storage conditions: expiration date is limited to the date listed by the manufacturer. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. for those materials where shelf life information is a requirement, expiration and retest periods are. Chemicals Expiry Date.
From www.thoughtco.com
Expiration Dates for Household Chemicals Chemicals Expiry Date a chemical might undergo chemical changes due to different storage conditions: expiration date is limited to the date listed by the manufacturer. It could react with other chemicals present in the air or in the. for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. the fda. Chemicals Expiry Date.
From www.weber.co.uk
GHS Chemical Labels CLP Compliant Labelling er UK Chemicals Expiry Date the fda has published a q&a document regarding the establishment of expiry dating for chemicals, reagents,. for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. a chemical might undergo chemical changes due to different storage conditions: expiration date refers to the amount of time an opened. Chemicals Expiry Date.
From outdoorindustry.org
Chemical Storage Chemicals Management Guide & Training for Manufacturers 1 Chemicals Expiry Date expiration date is limited to the date listed by the manufacturer. for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. a chemical might undergo chemical changes due to different storage conditions: when there is low risk of product degradation due to delivery and storage, unlikely contamination,. Chemicals Expiry Date.
From www.onlinelabels.com
Getting Your Hazard Labels OSHA Ready Label Learning Center Chemicals Expiry Date expiration date is limited to the date listed by the manufacturer. for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. It could react with other chemicals present in. Chemicals Expiry Date.
From www.dreamstime.com
Manufacturing Date and Expiry Date on Some Pharmaceutical Packaging. Stock Photo Image of Chemicals Expiry Date for those materials where shelf life information is a requirement, expiration and retest periods are available at the batch. expiration date refers to the amount of time an opened reagent will last before needing to be disposed of. when there is low risk of product degradation due to delivery and storage, unlikely contamination, and availability of simple. Chemicals Expiry Date.